Participants
Participants were recruited between August 2023 and the time of statistical analysis through offline advertisements at Beijing Huilongguan Hospital. A total of 22 individuals with schizophrenia and 22 healthy controls matched for gender, age, and educational level were initially enrolled. Due to excessive head motion (translation > 3 mm or rotation > 3°), imaging data from 2 patients and 1 control were excluded. Ultimately, data from 20 patients and 21 healthy controls were included in the final analysis.
The diagnosis of schizophrenia was confirmed by two experienced licensed psychiatrists based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Patients met the diagnostic criteria for schizophrenia and did not meet criteria for any other major psychiatric disorders, including neurodevelopmental disorders, other psychotic disorders, bipolar and related disorders, depressive disorders, trauma- and stressor-related disorders, substance-related and addictive disorders, neurocognitive disorders.
Healthy controls and their first-degree relatives were assessed to ensure no current or past diagnosis of any major psychiatric disorder according to DSM-5 criteria, including neurodevelopmental disorders, schizophrenia spectrum and other psychotic disorders, bipolar and related disorders, depressive disorders, trauma- and stressor-related disorders, substance-related and addictive disorders, neurocognitive disorders,
All participants met the following inclusion criteria: (1) right-handedness confirmed by the Edinburgh handedness inventory; (2) aged 18–55 years without gender restriction; (3) at least junior high school education with normal intelligence and ability to comprehend and complete study-related assessments and procedures; (4) absence of severe or unstable physical illness, particularly seizure-related conditions, such as epilepsy or other disorders that may induce seizures (e.g., neurological diseases or severe head trauma); (5) no contraindications for MRI scanning, including electronic/metal implants (e.g., pacemakers, neurostimulators, implanted pumps, cochlear implants, vascular clips, metallic prostheses, or permanent eyeliner) and no history of claustrophobia; (6) absence of comorbid known or suspected borderline or antisocial personality disorders, or any other psychiatric conditions of sufficient severity to interfere with study participation..
This study was approved by the Ethics Committee of Beijing Huilongguan Hospital. All participants and their legal guardians were fully informed of the purpose and procedures of the study and voluntarily provided written informed consent. To minimize the risk of coercion, informed consent was obtained by research staff rather than psychiatric clinicians involved in patients’ treating. All study procedures have been performed in accordance with the Declaration of Helsinki.
Action-outcome contingent paradigm
The effect of sensory attenuation is frequently assessed using ERP techniques combined with the action-outcome contingent paradigm. Typically, this paradigm consists of three core experimental conditions to systematically examine the interaction between action execution and sensory feedback 27:
-
(1)
Action-outcome condition Participants are instructed to voluntarily perform a repetitive action, such as pressing a button, tapping a key, or moving a finger. Each action elicits a perceptible sensory consequence, such as auditory feedback.
-
(2)
Stimulus-only condition Participants remain still and passively receive a series of stimuli without executing movements. The effect of sensory attenuation is generally defined as the differences of ERPs between action-outcome and stimulus-only conditions 28.
-
(3)
Action-only condition A supplementary control condition employed in prior ERP studies, where participants voluntarily repeat actions as in the action-outcome condition but receive no contingent sensory outcomes. The ERPs recorded in this condition are subtracted from those in the action-outcome condition to isolate action-contingent sensory modulation 28.
Although the action-outcome contingent paradigm holds significant value in sensory attenuation research, the application of this classical design requires consideration of its methodological limitations. Traditionally, two key design aspects require refinement:
First, significant cognitive load imbalance exists between experimental conditions. Specifically, both the action-outcome and action-only conditions require participants to actively perform designated actions. In contrast, the stimulus-only condition involves purely passive stimulus reception. This asymmetry of task may lead to differences in attentional resource between conditions. Consequently, systematic bias may be introduced into the comparison of neural activity across conditions. Therefore, recent studies have improved condition comparability. Cognitive operational complexity has been balanced across conditions, and attention-monitoring tasks (e.g., oddball detection task) have been added 29,30.
Second, the paradigm’s homogeneity assumption regarding the effects of action lacks sufficient validation. Its core presupposition is that the motor execution process is neurophysiologically equivalent in the action-outcome and action-only conditions. However, supporting evidence remains insufficient. Direct validation of motor cortex activity consistency between these conditions using electrophysiological techniques is lacking. Furthermore, empirical data comparing neuroimaging signatures of sensory attenuation-related brain regions using fMRI are also scarce. This gap between theoretical presupposition and empirical foundation may compromise the validity of causal inferences regarding the neurofunctional characteristics of sensory attenuation 28.
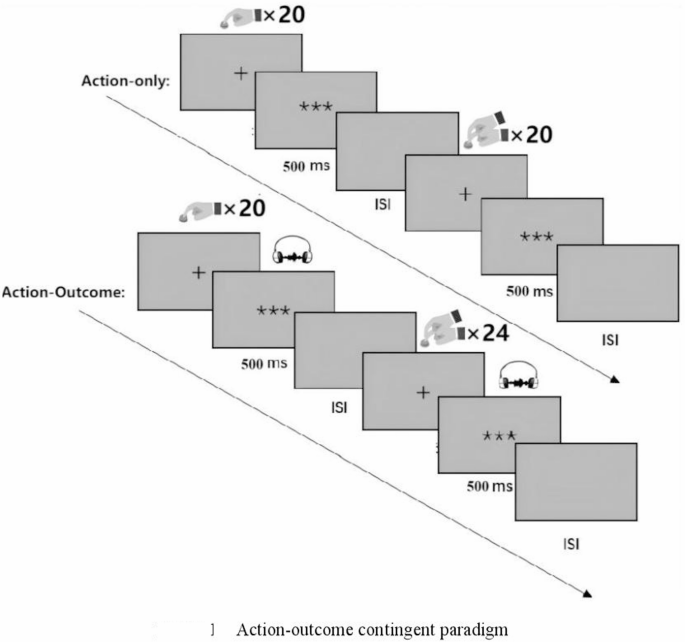
To address these limitations, the present study implemented a modified action-outcome contingent paradigm wherein the traditional stimulus-only condition was replaced with a passive button-press condition (Fig. 1). In this condition, participants were instructed to remain still and keep their right index finger on the button while the experimenter manually pressed participants’ right index finger, thus maintaining biomechanical equivalence while eliminating voluntary action components. This methodological adaptation optimizes control over confounding action-related variables between active and passive conditions.
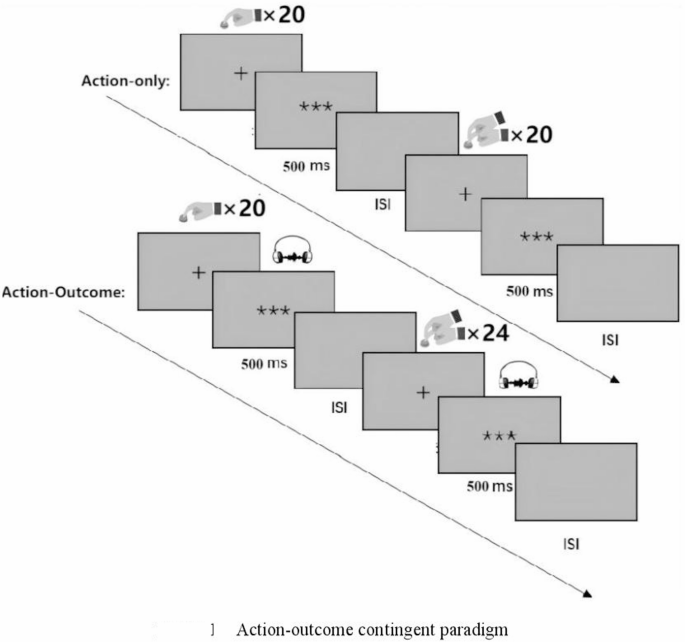
Action-outcome contingent paradigm.
Consequently, the formal experimental protocol comprised two counterbalanced conditions:
-
(1)
Active condition Participants were instructed to press a button using their right index finger at a self-chosen moment (typically within 4 s) following the appearance of a fixation cross (“ + ”). This temporal constraint was designed to elicit a well-prepared, self-initiated action rather than a direct response to the fixation cue.
-
(2)
Passive condition Participants were required to keep their finger stationary. The researcher then manually pressed the participant’s right index finger to complete the key press within the same time window (within 4 s after fixation onset). Autonomous motor intention was thereby eliminated, effectively distinguishing biological effects between intentional modulation and mechanical movement.
Critically, a staged experimental design was employed to precisely dissociate neural responses to spontaneous versus externally triggered sensory inputs. This design facilitated the investigation of sensory attenuation while controlling for confounding motor variables:
The first stage specifically examined neurofunctional characteristics under the action-only condition to clarify confounding effects of motor variables. Experimental validity was ensured through the following core designs:
-
(1)
Two movement modes were established, participants either actively pressed a button with their right index finger or received passive finger movements administered by an experimenter (20 trials each). Each trial lasted 6 s, followed by a 4-s rest period, totaling 6 min and 40 s. Additionally, the order of active and passive conditions was randomized across participants to balance the effects of sequence.
-
(2)
The auditory feedback channel was completely blocked throughout the action-only stage. Moreover, this stage was consistently implemented before the action-outcome stage to eliminate the potential influence of anticipatory outcome effects on neural activity in the action-only condition. This was crucial because, in the action-outcome condition, button-presses (both active and passive) were associated with sensory consequences.
The subsequent stage specifically examined neurofunctional characteristics under the action-outcome condition. This examination aimed to contrast neural processing differences between self-initiated and passively triggered stimuli. Therefore, a standardized feedback mechanism was established to enhance result comparability. Specifically, a precisely time-locked system was implemented for both conditions: Following button-press triggering, an acoustic stimulus (0.5 kHz pure tone or 1 kHz white noise) was presented within a ≤ 1 ms time window and persisted for 500 ms. Furthermore, all acoustic stimuli were delivered via a customized MRI-compatible audio system.
Consistent with the action-only condition, an active–passive dual-condition paradigm was also constructed for the action-outcome condition to enhance research validity. Specifically, the experiment comprised 44 trials (20 active + 24 passive). A between-subject randomization strategy was adopted for order balancing. Consequently, systematic interference from the sequence of active/passive conditions on research was prevented. Moreover, the settings of temporal parameter were aligned with the action-only condition. Each trial lasted 6 s, followed by a 4-s inter-trial rest period. Total experimental duration was controlled at 7 min and 20 s. Thus, by unifying temporal parameters and dual-condition paradigm of movement modes, interference from extraneous variables was effectively controlled. Differences in experimental effects could therefore be attributed to the core characteristics of the action execution mode.
To address the imbalance of attentional load in the passive condition, participants were informed beforehand to perform an oddball detection task (500 ms, 1 kHz noise) during the passive phase. Operationally, four oddball stimuli (1 kHz white noise, 500 ms) were randomly triggered by four button-presses, and participants were required to press a button using their left index finger upon detecting the oddball stimuli. Attention levels were thereby effectively maintained. It should be noted that all trials containing oddball stimuli were excluded from subsequent data analysis.
Acquisition and preprocessing of fMRI data
All task-based fMRI data were acquired at Beijing Huilongguan Hospital Neuroimaging Center using a 3.0 T Siemens Prisma scanner with a 12-channel head coil. Whole-brain coverage was achieved via gradient-echo planar imaging (EPI) sequence with 32 axial slices (5 mm thickness) acquired in ascending interleaved acquisition (foot-to-head, slice-skipping). Imaging parameters included: voxel size = 3.8 × 3.8 × 5 mm3, field of view = 240 mm, repetition time = 2000 ms, echo time = 30 ms, flip angle = 70°. Each participant completed 84 trials yielding 420 whole-brain EPI images across a 14-min scanning session.
To facilitate co-registration of functional and structural images, high-resolution T1-weighted structural images were obtained using a magnetization-prepared rapid gradient-echo sequence with the same scanner and identical scanner configuration: voxel size = 1 × 1 × 1 mm3, field of view = 256 mm, repetition time = 2000 ms, echo time = 2.28 ms, flip angle = 9°.
All fMRI data were preprocessed using RESTplus v1.30_20240508 (http://www.restfmri.net/forum/RESTplus), a task-based fMRI batch preprocessing toolkit built on SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12) and operated via MATLAB R2022b (https://www.mathworks.cn/products/matlab.html). The “Pipeline” module of RESTplus was used for batch preprocessing and the following preprocessing steps were applied:
-
(1)
Realignment EPI images were realigned to the median image of each time series to correct for slice-timing and head motion.
-
(2)
Coregistration Each participant’s T1-weighted structural images were coregistered to their corresponding mean EPI images.
-
(3)
Segmentation Gray matter, white matter, and cerebrospinal fluid were segmented using the New Segment toolbox in SPM12.
-
(4)
Normalization Segmented structural images were normalized to the Montreal Neurological Institute (MNI) standard space and the transformation was applied to the EPI images (resampled at 3 × 3 × 3 mm3 resolution). Systematic visual inspection was performed across all participants to verify registration accuracy and exclude spatial artifacts after normalization.
-
(5)
Smooth A 6 mm full-width half-maximum Gaussian kernel was applied to spatially smooth the normalized EPI images.
Statistical analysis
First, preprocessed EPI images were subjected to first-level analysis for each participant. Each experimental condition was modeled as an independent regressor, and six head motion parameters were included as nuisance covariates. All regressors were convolved with the canonical hemodynamic response function.
Second, a 2 (group: patients vs. controls) × 2 (condition: active vs. passive) mixed-design ANOVA was conducted to estimate main effects of group, main effects of condition, and group × condition interaction effects. For brain regions showing significant interaction effects, post-hoc analysis were performed to determine activation differences between groups and conditions. The clusters with significant interactions were then defined as seed regions (6-mm radius spheres) for psychophysiological interaction (PPI) analysis, which aimed to explore condition-dependent (active − passive) FC differences. Independent samples t-tests were used to assess between-group differences in FC. Statistical significance for blood oxygen level dependent signal time courses was assessed at cluster-level threshold of family-wise error (FWE) correction, P < 0.05.
Finally, the interaction-significant clusters were subsequently defined as regions of interest (ROIs, 6-mm radius spheres) to extract the activation difference between conditions (passive − active) for each schizophrenia patient. These values were correlated with clinical variables including illness duration, chlorpromazine equivalents dose, positive and negative syndrome scale (PANSS) scores (total score, and positive/negative/general psychopathology scores) using SPSS 22.0 (https://www.ibm.com/spss).
For each result, the peak voxel coordinates (x, y, z in MNI space), cluster size (number of voxels), the multiple comparison correction method, and statistical values (t or F values) were reported. All imaging results were visualized using MRIcroGL (https://www.nitrc.org/projects/mricrogl/).