Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–55.
Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes, Metab Syndr Obes. 2020;13:3611–6.
Bartlett DB, Slentz CA, Willis LH, Hoselton A, Huebner JL, Kraus VB, et al. Rejuvenation of neutrophil functions in association with reduced diabetes risk following ten weeks of low-volume high intensity interval walking in older adults with prediabetes–a pilot study. Front Immunol. 2020;11:729.
Grohová A, Dáňová K, Špíšek R, Palová-Jelínková L. Cell based therapy for type 1 diabetes: should we take hyperglycemia into account?. Front Immunol. 2019;10:79.
Rodriguez-Sosa M, Cabellos-Avelar T, Sanchez-Zamora Y, Juarez-Avelar I, Garcia-Reyes E, Lira-Leon A, et al. Proinflammatory cytokine MIF plays a role in the pathogenesis of type-2 diabetes mellitus, but does not affect hepatic mitochondrial function. Cytokine. 2017;99:214–24.
Chang TT, Li YZ, Mo HW, Chen C, Lin LY, Chang CC, et al. Inhibition of CCL7 improves endothelial dysfunction and vasculopathy in mouse models of diabetes mellitus. Sci Transl Med. 2024;16:eadn1507.
Perez N, He N, Wright F, Condon E, Weiser S, Aouizerat B. Social determinants of inflammatory markers linking depression and type 2 diabetes among women: a scoping review. J Psychosom Res. 2024;184:111831.
Rashed AA, Saparuddin F, Rathi D-NG, Nasir NNM, Lokman EF. Effects of resistant starch interventions on metabolic biomarkers in pre-diabetes and diabetes adults. Front Nutr. 2022;8:793414.
Wu Y, Ma Y. CCL2-CCR2 signaling axis in obesity and metabolic diseases. J Cell Physio. 2024;239:e31192.
Yousef H, Khandoker AH, Feng SF, Helf C, Jelinek HF. Inflammation, oxidative stress and mitochondrial dysfunction in the progression of type II diabetes mellitus with coexisting hypertension. Front Endocrinol. 2023;14:1173402.
Tagoma A, Haller-Kikkatalo K, Oras A, Roos K, Kirss A, Uibo R. Plasma cytokines during pregnancy provide insight into the risk of diabetes in the gestational diabetes risk group. J Diabetes Investig 2022;13:1596–606.
Qureshi N, Desousa J, Siddiqui AZ, Drees BM, Morrison DC, Qureshi AA. Dysregulation of gene expression of key signaling mediators in PBMCs from people with type 2 diabetes mellitus. Int J Mol Sci. 2023;24:2732.
Blériot C, Dalmas É, Ginhoux F, Venteclef N. Inflammatory and immune etiology of type 2 diabetes. Trends Immunol. 2023;44:101–9.
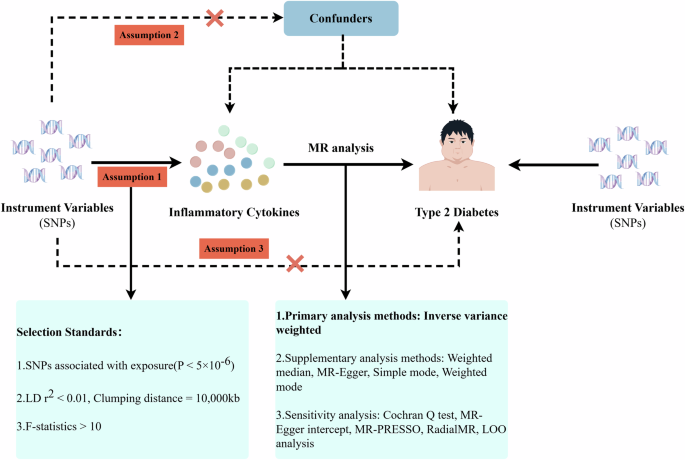
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafo MR, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2:6.
Xiang M, Wang Y, Gao Z, Wang J, Chen Q, Sun Z, et al. Exploring causal correlations between inflammatory cytokines and systemic lupus erythematosus: a Mendelian randomization. Front Immunol. 2022;13:985729.
Liu H, Liu Z, Huang Y, Ding Q, Lai Z, Cai X, et al. Exploring causal association between circulating inflammatory cytokines and functional outcomes following ischemic stroke: a bidirectional Mendelian randomization study. Eur J Neurol. 2024;31:e16123.
Zhang J, Li K, Qiu X. Exploring causal correlations between inflammatory cytokines and knee osteoarthritis: a two-sample Mendelian randomization. Front Immunol. 2024;15:1362012.
Yin Z, Chen J, Xia M, Zhang X, Li Y, Chen Z, et al. Assessing causal relationship between circulating cytokines and age-related neurodegenerative diseases: a bidirectional two-sample Mendelian randomization analysis. Sci Rep. 2023;13:12325.
Ahola-Olli AV, Würtz P, Havulinna AS, Aalto K, Pitkänen N, Lehtimäki T, et al. Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am J Hum Genet. 2017;100:40–50.
Shi Q, Wang Q, Wang Z, Lu J, Wang R. Systemic inflammatory regulators and proliferative diabetic retinopathy: A bidirectional Mendelian randomization study. Front Immunol. 2023;14:1088778.
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–13.
Yuan S, Li X, Liu Q, Wang Z, Jiang X, Burgess S, et al. Physical activity, sedentary behavior, and type 2 diabetes: Mendelian randomization analysis. J Endocr Soc. 2023;7:bvad090.
Yi M, Zhao W, Fei Q, Tan Y, Liu K, Chen Z, et al. Causal analysis between altered levels of interleukins and obstructive sleep apnea. Front Immunol. 2022;13:888644.
Zhang Z, Wang S, Ren F, Yang L, Xie H, Pan L, et al. Inflammatory factors and risk of meningiomas: a bidirectional mendelian-randomization study. Front Neurosci. 2023;17:1186312.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64.
Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet. 2021;53:156–65.
Wang J, Liu D, Tian E, Guo ZQ, Chen JY, Kong WJ, et al. Is hearing impairment causally associated with falls? Evidence from a two-sample Mendelian randomization study. Front Neurol. 2022;13:876165.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Georgakis MK, Gill D, Rannikmäe K, Traylor M, Anderson CD, Lee JM, et al. Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 2019;139:256–68.
Chen S, Zhou Y, Chen Y, Gu JJB. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018;34:i884–i890.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9.
Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–45.
Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–82.
Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12:1088.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377–89.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74.
Gilbert ER, Fu Z, Liu D. Development of a nongenetic mouse model of type 2 diabetes. Exp Diab Res. 2011;2011:416254.
Wang Y, Wang X, Jia X, Li J, Fu J, Huang X, et al. Influenza vaccination features revealed by a single-cell transcriptome atlas. J Med Virol. 2023;95:e28174.
Wang Y, Sun Q, Zhang Y, Li X, Liang Q, Guo R, et al. Systemic immune dysregulation in severe tuberculosis patients revealed by a single-cell transcriptome atlas. J Infect. 2023;86:421–38.
Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. 2018;564:268–72.
Zhang JY, Wang XM, Xing X, Xu Z, Zhang C, Song JW, et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol. 2020;21:1107–18.
Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020;181:442–59.e29.
Crinier A, Milpied P, Escaliere B, Piperoglou C, Galluso J, Balsamo A, et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK cell subsets in humans and mice. Immunity. 2018;49:971–86.e5.
Collier JL, Pauken KE, Lee CAA, Patterson DG, Markson SC, Conway TS et al. Single-cell profiling reveals unique features of diabetogenic T cells in anti-PD-1-induced type 1 diabetes mice. J Exp Med. 2023;220:e20221920.
Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12.
Guo H, Xu B, Gao L, Sun X, Qu X, Li X, et al. High frequency of activated natural killer and natural killer T-cells in patients with new onset of type 2 diabetes mellitus. Exp Biol Med. 2012;237:556–62.
O’Rourke RW, Gaston GD, Meyer KA, White AE, Marks DL. Adipose tissue NK cells manifest an activated phenotype in human obesity. Metabolism. 2013;62:1557–61.
Viel S, Besson L, Charrier E, Marcais A, Disse E, Bienvenu J, et al. Alteration of Natural Killer cell phenotype and function in obese individuals. Clin Immunol. 2017;177:12–7.
Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB. Are natural killer cells protecting the metabolically healthy obese patient?. Obesity. 2009;17:601–5.
Bahr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. 2018;66:234–44.
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T, et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab. 2016;23:685–98.
O’Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M, et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity. 2014;22:2109–14.
Yoon Kim D, Kwon Lee J. Type 1 and 2 diabetes are associated with reduced natural killer cell cytotoxicity. Cell Immunol. 2022;379:104578.
Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87.
Tang L, Wang H, Cao K, Xu C, Ma A, Zheng M, et al. Dysfunction of circulating CD3(+)CD56(+) NKT-like cells in type 2 diabetes mellitus. Int J Med Sci. 2023;20:652–62.
Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17.
Mahlangu T, Dludla PV, Nyambuya TM, Mxinwa V, Mazibuko-Mbeje SE, Cirilli I, et al. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine. 2020;126:154892.
Sarikonda G, Pettus J, Phatak S, Sachithanantham S, Miller JF, Wesley JD, et al. CD8 T-cell reactivity to islet antigens is unique to type 1 while CD4 T-cell reactivity exists in both type 1 and type 2 diabetes. J Autoimmun. 2014;50:77–82.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20.
Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32:451–63.
Nojima I, Eikawa S, Tomonobu N, Hada Y, Kajitani N, Teshigawara S, et al. Dysfunction of CD8 + PD-1 + T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci Rep. 2020;10:14928.
Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–7.
Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9.
Srikakulapu P, McNamara CA. B lymphocytes and adipose tissue inflammation. Arterioscler Thromb Vasc Biol. 2020;40:1110–22.
DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci USA. 2013;110:5133–8.
Simar D, Versteyhe S, Donkin I, Liu J, Hesson L, Nylander V, et al. DNA methylation is altered in B and NK lymphocytes in obese and type 2 diabetic human. Metabolism. 2014;63:1188–97.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–74.
Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Arch Pharm Res. 2013;36:208–22.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84.
Seifarth CC, Hinkmann C, Hahn EG, Lohmann T, Harsch IA. Reduced frequency of peripheral dendritic cells in type 2 diabetes. Exp Clin Endocrinol Diab. 2008;116:162–6.
Musilli C, Paccosi S, Pala L, Gerlini G, Ledda F, Mugelli A, et al. Characterization of circulating and monocyte-derived dendritic cells in obese and diabetic patients. Mol Immunol. 2011;49:234–8.
Pecht T, Haim Y, Bashan N, Shapiro H, Harman-Boehm I, Kirshtein B, et al. Circulating blood monocyte subclasses and lipid-laden adipose tissue macrophages in human obesity. PLoS One. 2016;11:e0159350.
Devevre EF, Renovato-Martins M, Clement K, Sautes-Fridman C, Cremer I, Poitou C. Profiling of the three circulating monocyte subpopulations in human obesity. J Immunol. 2015;194:3917–23.
Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820.
Kochetova OV, Avzaletdinova DS, Morugova TV, Mustafina OE. Chemokine gene polymorphisms association with increased risk of type 2 diabetes mellitus in Tatar ethnic group, Russia. Mol Biol Rep. 2019;46:887–96.
Lu X, Wang Z, Ye D, Feng Y, Liu M, Xu Y et al. The role of CXC chemokines in cardiovascular diseases. Front Pharmacol. 2022;12:765768.
Matsushita Y, Hasegawa Y, Takebe N, Onodera K, Shozushima M, Oda T, et al. Serum C-X-C motif chemokine ligand 14 levels are associated with serum C-peptide and fatty liver index in type 2 diabetes mellitus patients. J Diab Investig. 2021;12:1042–9.
Chen L, Yang Z, Lu B, Li Q, Ye Z, He M, et al. Serum CXC ligand 5 is a new marker of subclinical atherosclerosis in type 2 diabetes. Clin Endocrinol. 2011;75:766–70.
Mir MM, Alfaifi J, Sohail SK, Rizvi SF, Akhtar MT, Alghamdi MAA et al. The role of pro-inflammatory chemokines CCL-1, 2, 4, and 5 in the etiopathogenesis of type 2 diabetes mellitus in subjects from the Asir Region of Saudi Arabia: correlation with different degrees of obesity. J Pers Med. 2024;14:743.
Chan PC, Hung LM, Huang JP, Day YJ, Yu CL, Kuo FC, et al. Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity. Clin Sci. 2022;136:121–37.
Zhou H, Liao X, Zeng Q, Zhang H, Song J, Hu W, et al. Metabolic effects of CCL5 deficiency in lean and obese mice. Front Immunol. 2022;13:1059687.
Liang YC, Jia MJ, Li L, Liu DL, Chu SF, Li HL. Association of circulating inflammatory proteins with type 2 diabetes mellitus and its complications: a bidirectional Mendelian randomization study. Front Endocrinol. 2024;15:1358311.
Zhao JH, Stacey D, Eriksson N, Macdonald-Dunlop E, Hedman AK, Kalnapenkis A, et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol. 2023;24:1540–51.
Chen Z, Jiang G, Jiang G, Ma S, Zhu Y, Zhao M. Circulating inflammatory cytokines and gestational diabetes mellitus: Unraveling the role of macrophage migration inhibitory factor (MIF) through a bidirectional mendelian randomization study. Cytokine. 2024;182:156734.
Uchi H, Terao H, Koga T, Furue M. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24:S29–38.
Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24:18–27.
Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–22.










Leave feedback about this